
PIPELINE DMD
Delivering on the RNA Revolution
We are advancing and expanding our innovative AOC pipeline to offer treatment options for patients and their families across a wide range of therapeutic areas. We have three AOC programs for three distinct rare diseases in clinical development from our muscle disease franchise: myotonic dystrophy type 1 (DM1), facioscapulohumeral muscular dystrophy (FSHD), and Duchenne Muscular Dystrophy (DMD). Our pipeline also includes advancing AOCs to address additional DMD, rare neuromuscular, and rare precision cardiology programs. We continue to broaden our reach of AOCs in other indications including cardiology and immunology through partnerships.
Duchenne Muscular Dystrophy
Duchenne muscular dystrophy (DMD) is a rare, genetic condition that is characterized by progressive muscle damage and weakness. It is caused by a genetic mutation that prevents the body from producing a protein called dystrophin, which is an important protein that protects muscle cells from injury during contraction. The lack of functional dystrophin leads to stress and tears of muscle cell membranes, resulting in muscle cell death and progressive loss of muscle function. Those living with the condition often require special aid and assistance throughout their lives and have significantly shortened life expectancy. DMD is a monogenic, X-linked, recessive disease that primarily affects males, with 1 in 3,500 to 5,000 boys born worldwide having Duchenne.
We are developing AOCs to treat the underlying cause of DMD. The oligonucleotides in our AOCs for treatment of DMD are designed to promote the skipping of specific exons to allow the production of the dystrophin gene product in patients with DMD.
In preclinical studies, we observed that treatment of an mdx mouse, a widely used animal model for DMD, with an AOC caused a greater than 50-fold increase in exon skipping compared to an equimolar dose of the unconjugated oligonucleotide.
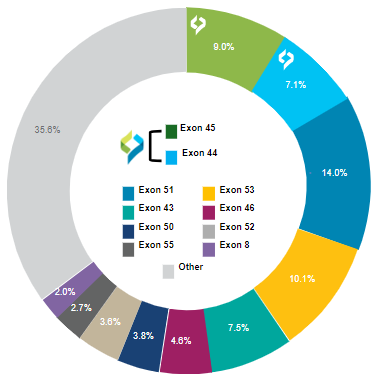
DMD Exons Amenable to Skipping:
Delpacibart zotadirsen or del-zota
Del-zota is designed to deliver phosphorodiamidate morpholino oligomers (PMOs) to skeletal muscle and heart tissue to specifically skip exon 44 of the dystrophin gene to enable dystrophin production in people living with Duchenne muscular dystrophy with mutations amenable to exon 44 skipping (DMD44). DMD is characterized by progressive muscle degeneration and weakness due to alterations of a protein called dystrophin that protects muscle cells from injury during contraction. Del-zota consists of a proprietary monoclonal antibody that binds to the transferrin receptor 1 (TfR1) conjugated with a PMO targeting exon 44. The Phase 1/2 EXPLORE44® trial of del-zota has been completed, and the EXPLORE44 Open-Label Extension trial (OLE™) of del-zota is currently ongoing. Topline data from the completed del-zota Phase 1/2 EXPLORE44 trial demonstrated unsurpassed delivery of PMOs to skeletal muscle, robust increases in dystrophin production, significant increases in exon 44 skipping, and significant and sustained decreases of creatine kinase levels to near normal in people living with DMD44. Del-zota has received Rare Pediatric Disease, Orphan Drug and Fast Track designations by the U.S. Food and Drug Administration (FDA) and Orphan designation by the European Medicines Agency (EMA).
Topline Del-zota data from EXPLORE44® clinical trial in People Living with DMD44 Presented in March 2025
In March 2025, Avidity announced positive topline del-zota data demonstrating consistent data across key measures in both the 5 mg/kg and 10 mg/kg cohorts of del-zota including statistically significant increase of approximately 25% of normal in dystrophin production, increases of approximately 40% in exon 44 skipping, and reduction of creatine kinase levels to near normal with greater than 80% reductions compared to baseline. Del-zota also demonstrated favorable safety and tolerability at both doses, with most treatment emergent adverse events (TEAEs) mild or moderate.
Based on the consistent data between the 5 mg/kg every six weeks and the 10mg/kg every eight weeks groups, Avidity has selected the dose of 5 mg/kg every six weeks of del-zota for the Biologics License Application (BLA) submission and future clinical studies. Participants currently receiving the 10 mg/kg dose in the EXPLORE44-OLE™ trial are in the process of being transitioned to 5 mg/kg every six weeks.
Initial 5 mg/kg Del-zota data for People Living with DMD44 Presented in August 2024
In August 2024, Avidity announced positive del-zota 5 mg/kg data demonstrating unsurpassed delivery of phosphorodiamidate morpholino oligomers (PMO) concentrations to skeletal muscle, statistically significant increase of 25% of normal in dystrophin production and statistically significant increase of 37% in exon 44 skipping in the Phase 1/2 EXPLORE44™ clinical trial. Del-zota 5 mg/kg reduced creatine kinase levels to near normal with greater than 80% reduction compared to baseline.

The Phase 1/2 EXPLORE44® trial is now complete. The study was a randomized, placebo-controlled, double-blind, Phase 1/2 clinical trial to evaluate del-zota in healthy volunteers and participants living with DMD44. EXPLORE44 evaluates the safety, tolerability, pharmacokinetics, and pharmacodynamic effects of single and multiple ascending doses of del-zota administered intravenously, and assesses exon skipping and dystrophin protein levels in participants with DMD44. Participants in the EXPLORE44 trial have the option to enroll into the open-label extension study, EXPLORE44-OLE™. For more information about the EXPLORE44 trial, visit Avidity’s website publications page, the EXPLORE44 study website or visit https://www.clinicaltrials.gov and search for NCT05670730.

The Phase 2 EXPLORE44-OLE™ trial is an open-label, multi-center trial designed to evaluate the long-term safety and tolerability of del-zota in participants living with DMD44. Participants in the EXPLORE44® trial had the option to enroll in the ongoing EXPLORE44-OLE study for del-zota. In addition, Avidity enrolled additional participants in the EXPLORE44-OLE study to support Avidity’s first BLA submission anticipated at year end 2025. The EXPLORE44-OLE study completed enrollment in February 2025 with a total of 39 participants enrolled. This trial will evaluate the safety, tolerability, PK, PD, and efficacy of del-zota. For more information on this study click here or visit https://www.clinicaltrials.gov and search for NCT06244082.