Platform Overview
Delivering on the RNA Revolution
Antibody Oligonucleotide Conjugates (AOC) therapeutics are designed to combine the proven technology of monoclonal antibodies (mAbs) with the precision and potency of oligonucleotide therapies to access previously untreatable tissue and cell types, opening new potential treatments for patients. We have done the experiments and have followed the data to build our AOC platform. We are revolutionizing the RNA therapeutics field by delivering oligonucleotides where they are needed – including beyond the liver.
Utilizing our proprietary Antibody Oligonucleotide Conjugates (AOCs™) platform, Avidity was the first company to demonstrate the successful targeted delivery of RNA to muscle. This groundbreaking accomplishment in the RNA field has led to unprecedented and consistent data across all three of its clinical development programs for three different types of rare muscle diseases: myotonic dystrophy type 1 (DM1), facioscapulohumeral muscular dystrophy (FSHD) and Duchenne muscular dystrophy amenable to exon 44 skipping (DMD44). We continue to advance early-stage development programs in skeletal muscle and precision cardiology, a new therapeutic field to address the root cause of genetic diseases of the heart.
Our proprietary AOC™ platform expands the ability to address targets and diseases previously unreachable with existing RNA therapies. It is built from years of in-house engineering that integrates oligonucleotide therapeutics, modulation of RNA processes, antibody engineering and conjugation, and advanced drug delivery techniques.
We followed the data to engineer each component of our AOCs:
Components
Characteristics
Monoclonal

Approved mAbs offer:
- Well-established safety profiles
- High specificity and affinity
- Long half-lives
- Designed through engineering to be effector function null
- Epitope selection designed for optimal activity
Linker

- Known linker
- Applicable to multiple oligo modalities
- Enhanced for durability
- Engineered sites of conjugation
- Optimized ratio of oligonucleotides to antibodies
siRNA

Approved siRNA drugs have shown:
- Attractive safety profiles – no known thrombocytopenia, liver or renal toxicity
- Potency in the nanomolar range
- Sustained activity in the cytoplasm and nucleus
- Engineered to withstand lysosomal enzymes
- Selected and modified to diminish off-target effects
PMO

Approved PMO drugs have shown:
- Attractive safety profile
- Potency in the nanomolar range
- Sustained activity
- Engineered for efficient delivery to muscle – increased drug to antibody ratio
The flexibility of our AOC platform allows us to deploy various types of oligonucleotides, including small interfering RNAs (siRNAs) and phosphorodiamidate morpholino oligomers (PMOs), each of which modify RNA function in different ways. This allows us to use oligonucleotides that are tailored to modulate specific disease processes. Mechanisms of these oligonucleotides can range from reducing the expression of a disease-related RNA with siRNAs to correction of aberrant processing of RNAs with splice-modifying oligonucleotides.
OUR AOCS OFFER DISTINCT ADVANTAGES
We believe that the product candidates derived from our AOC platform will have the potential to impact a diverse set of diseases with the following advantages:
Expanding Scope of
Diseases Beyond Liver
Targeting new tissue and cell types, including muscle, immune cells and others
Selecting Most Potent
Oligonucleotide Type
Achieving ED50s at the nanomolar concentration
Enabling
Infrequent Dosing
Maximizing durability with sustained single dose RNA reductions in NHP beyond 12 weeks
Readily Reproducible
and Scalable
Utilizing the same monoclonal antibody across multiple programs
Beginning with our rare neuromuscular disease franchise, our platform targets the underlying genetic cause of disease — from here our deep pipeline continues to advance and expand into additional cells and tissues, including research and development programs in cardiology and immunology.
Some of these programs are in development based on research collaborations while others are based on internal discovery efforts. We look forward to pursuing all of these programs as we work to revolutionize the delivery of RNA therapeutics to profoundly improve people’s lives.
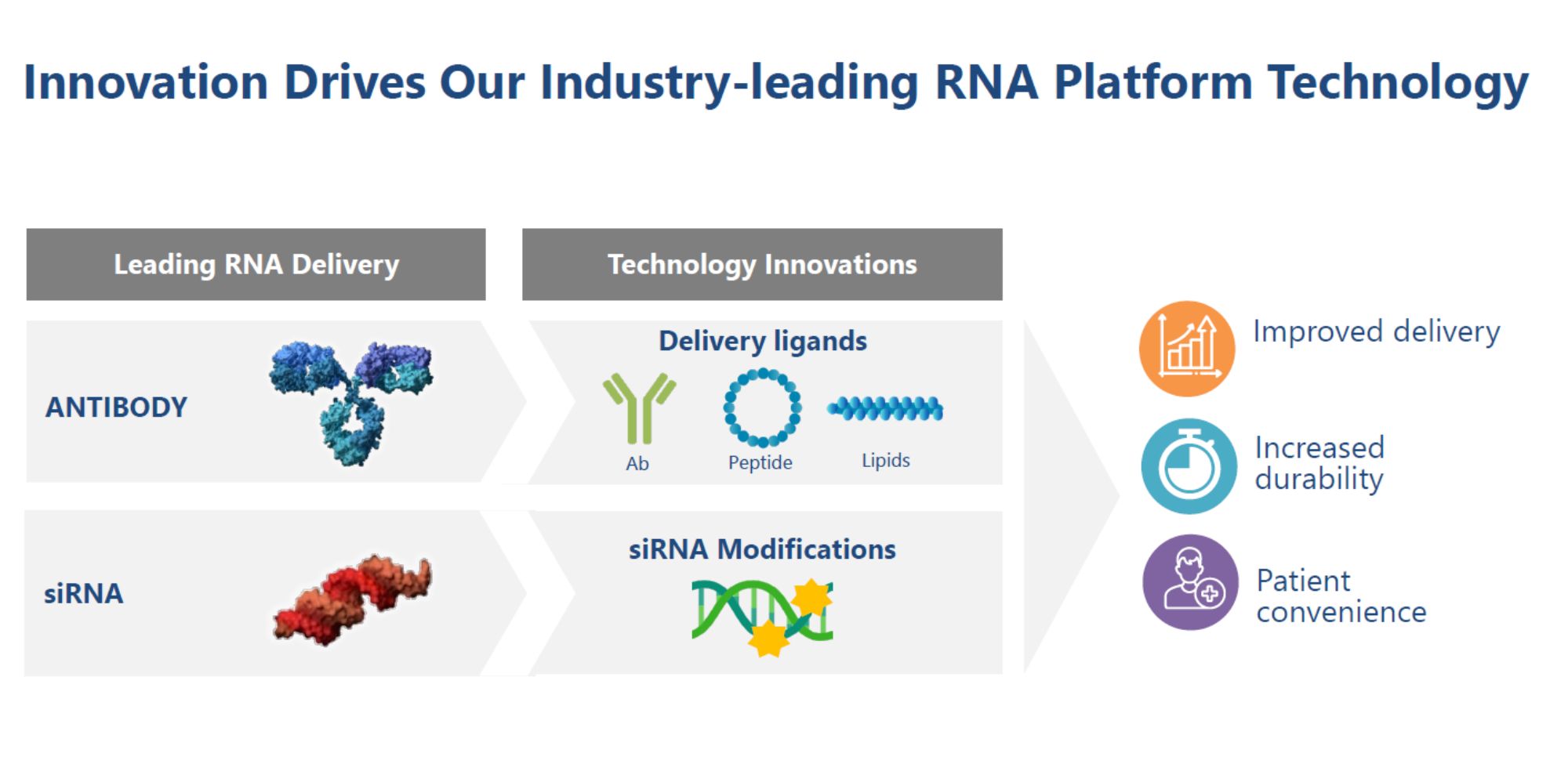
Next Generation Technology Innovations
Avidity continues to advance its RNA delivery technology with next-generation innovations, including siRNA modifications and evolved antibody engineering. In preclinical studies, Avidity’s leading RNA delivery technology provided up to 30-fold increase in siRNA delivery in skeletal muscle, and greater durability with sustained target inhibition for three months. Advancements in siRNA delivery and greater durability allow the opportunity for less frequent dosing and improved patient convenience.